3 Types of pressure and units from around the world
30 May 2022
What is pressure?
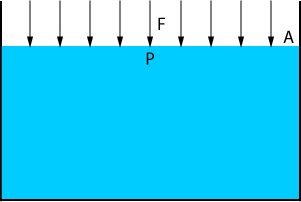
By definition, pressure is described as the amount of force applied perpendicular to a surface per unit of area.
It can be calculated by the following formula:
P = F A
| where: | P | = | Pressure |
| F | = | The resultant force | |
| A | = | The surface subjected to the force |
How is pressure physically created?
One way to look at pressure is to see it as the result of the weight of all stacked molecules on top of a surface. This approach fits best for solids and liquids.
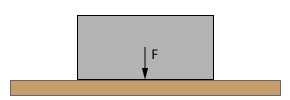
The figure above is showing a surface with a solid block on top of it.
Every molecule of that block has weight because gravity is pulling on it. Since weight is a downward force, every molecule will exert a small force on the surface.
The resultant force of all these small forces is creating the pressure.
When using this approach for gases, one could argue that the gas molecules are not stacked since they are freely floating around. So, how can they exert a force on that surface?
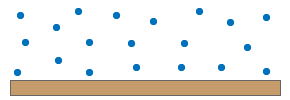
The molecules of a gas are in constant movement. As they move, they have momentum and kinetic energy. Frequently they will collide with each other and with the surface of an object.
With every collision with the surface, the molecules pass on momentum to that surface. This generates a force perpendicular to that surface.
The sum of the forces of all these colliding molecules is creating the pressure.
What are the different types of pressure?
There are three types of pressure:
- absolute pressure
- gauge pressure
- differential pressure
The difference between these three is the reference point that is chosen as the zero point on the scale. For absolute pressure, the perfect vacuum was chosen as the reference point, while for gauge pressure the reference point is atmospheric pressure. For differential pressure, there is no fixed reference point because two different pressures are compared.
The following drawing shows the different types of pressure. The starting point of each arrow coincides with a chosen reference point. Note that absolute pressure and differential pressure are always positive, while relative (gauge) pressure can also be slightly negative. In the latter case, we also call it a partial vacuum. Theoretically, the maximum partial vacuum is -1,013 bar gauge, which corresponds to a perfect vacuum.
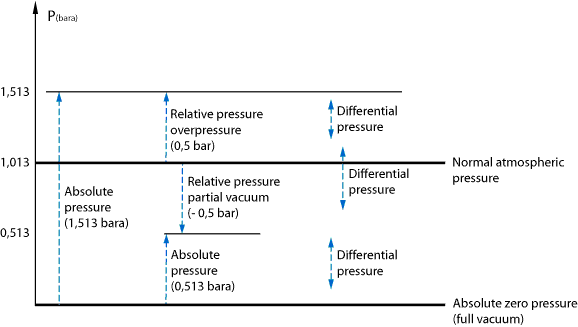
A pressure measurement is, in principle, always comparing the pressures between two different places.
For absolute pressure, the comparison is made between a location at a certain pressure and another location at absolute vacuum.
Likewise for relative (gauge) pressure where the comparison will be done with a location at normal atmospheric pressure (1013 mbar at sea level).
The measurement of differential pressure is comparing the pressures between two random locations.
Pressure measurement devices are specially designed for measuring these three different types of pressure and can, therefore, be classified accordingly.
Absolute pressure
Measuring something is done by comparing it to a well-known point of reference. For absolute pressure, the point of reference is the perfect vacuum. This point has been chosen because it is the lowest possible pressure. In particular, there is no pressure at all.
A perfect vacuum would mean that all particles have been removed from a closed volume. In this volume, which is then completely empty, pressure cannot be present.
As already said, absolute pressure is always a positive number. Negative numbers are impossible because there is no pressure below the perfect vacuum.
Gauge pressure (relative pressure)
Instead of comparing the measured pressure to a perfect vacuum we will now compare it to the standard atmospheric pressure at sea level. The latter amounts to 1013.25 mbar (14.696 psi).
The difference between absolute and gauge pressure, simultaneously measured at the same place, is always about 1 bar (14.50 psi).
Gauge pressure, sometimes also called relative pressure, can assume both positive and negative values. For positive
Po = Pabs – Patm
If the measured gauge pressure is negative, it is called underpressure or partial vacuum. The measured pressure is then lower than the standard atmospheric pressure and is found by subtracting the absolute pressure from the atmospheric pressure.
Pu = Patm – Pabs
By mentioning that it is a partial vacuum we do not need to use the minus sign. If a vacuum cleaner operates at an absolute pressure of 0,8 bar, we could also say that it operates at 0,2 bar
Differential pressure
Sometimes it is necessary to measure the pressure difference between two different points. When one nor the other point is a reference point, e.g. a perfect vacuum or the standard atmospheric pressure, it is called a differential pressure.
In theory, one could argue that absolute and gauge pressures are as
For example, 3 bar differential pressure between points A and B doesn’t say anything about the amount of pressure at points A and B, nor does it say anything about which point is at the highest pressure.
Are there any other types of pressure?
All pressure types we have discussed so far are based on the choice between the two conventional reference points or the comparison between two pressures.
However, there are certain kinds of pressure that are given a specific name to indicate the meaning of the pressure. Some examples of common pressures are:
- Vacuum pressure
- Atmospheric pressure
- Hydrostatic pressure
- Dynamic pressure
This has nothing to do with their relationship to a certain type of pressure since they can all be expressed as one of the three types of pressure.
So, no there are no other types. There are only other pressures with a specific name.
Below is a description of these common specific pressures.
Vacuum pressure
Strictly speaking, a vacuum is a space where the absolute pressure is zero. This can only be achieved if all particles are removed from that space. In other words, the space is really empty. The perfect vacuum is only theoretically possible. It will never be technically possible to remove all the particles in a closed volume.
A vacuum doesn’t need to be perfect to be called a vacuum. In practice, a vacuum will only be partially achieved. It is therefore also referred to as a partial vacuum. In general, we speak about a vacuum when the pressure is lower than the atmospheric pressure.
A high vacuum means that the absolute pressure is very low.
In order to create a vacuum, use is made of a vacuum pump. With this pump, the particles present inside a closed volume will be sucked out as much as possible. The capacity of the vacuum pump determines the level of the vacuum.
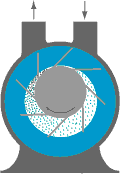
An example of a vacuum pump, which is used quite a lot in the industry, is the liquid ring vacuum pump. An eccentric impeller rotates in a pump housing, without making contact with this casing. Water is injected into the pump housing but insufficient to completely fill the pump. By centrifugal
Atmospheric pressure
The atmospheric
The atmospheric pressure, however, is not a constant but a variable value. The conditions in the atmosphere of our Earth are constantly changing. Influenced by the sun the air heats up, at night it cools off again. The humidity varies with the weather. The density of the air changes by high or
For the measurement of gauge pressure, this leads to a problem because the pressure to be measured is compared to the atmospheric pressure.
To obtain an unambiguous measurement of the gauge pressure, a standard atmospheric pressure has been introduced. As a reference, the average atmospheric pressure at sea level has been chosen, which meets the following conditions:
| Expressed in SI units |
|---|
| Patm = 1013,25 mbara |
| t = 15 °C |
| ρ = 1,226 kg/m³ |
| r = 287,1 J/(kg K) |
| Expressed in customary units |
|---|
| Patm = 14,696 psia |
| t = 59 °F |
| ρ = 0,002377 slugs/ft³ |
| r = 1716,49 ft lb/slug °R |
| Patm : absolute pressure |
| t : temperature |
| ρ : density |
| r : specific gas constant |
Hydrostatic pressure
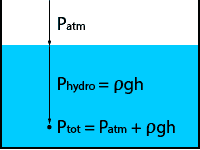
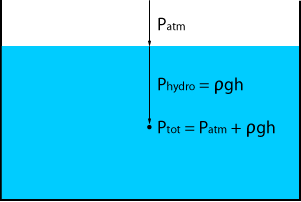
The term hydrostatic pressure is mainly used in fluids. It is the pressure at a given depth in the fluid caused by the weight of the column of liquid above it.
The hydrostatic pressure will depend on the density of the liquid, the gravitational constant and the height of the liquid column.
Hydrostatic pressure is a gauge pressure type and can be calculated with the following formula:
Phydro = ρgh (relative pressure)
If we also take into account the atmospheric pressure above the liquid surface, we find the total pressure:
Ptot = Patm + ρgh (absolute pressure)
As the atmospheric pressure is now accounted for in the equation, we are referencing to a perfect vacuum and so the total pressure becomes an absolute pressure.
Dynamic pressure
Dynamic pressure is one of the terms of Bernoulli’s equation. For incompressible fluids, this equation says that for steady flow along a streamline, the sum of pressure energy, kinetic energy, and potential energy remains constant.
The dynamic pressure is that part of the equation which represents the kinetic energy.
It is a pressure that is created by the kinetic energy of the molecules of a fluid when flowing for example through a pipe.
Dynamic pressure can be expressed with the following formula:
q = 1 2 ρv2
| where: | q | = | Dynamic pressure |
| ρ | = | Fluid mass density | |
| v | = | Flow speed |
Pressure units across several continents
Around the world, pressure is expressed in different units.
In the European Union, we are using the SI system as a legal standard. All physical quantities of products should be in line with the European directive 80/181/EEC (EU Metric Directive) and expressed according to this system. In this way, the
In the United Kingdom, the psi (pounds per square inch) is still often used, with 14.5 psi ≈ 1 bar, but is now being switched more and more to the bar pressure unit. To the
In the United States, the psi is still the main unit for measuring pressure. Almost all pressure gauges indicate the pressure in pounds per square inch.
In Asia, especially the units MPa (megaPascal) and kg/cm² (kilograms per square
In the table below you will find a few other units and their conversion factors to kPa and bar.
| Units | kPa | bar |
| 1 kPa | 1 | 0,01 |
| 1 MPa | 1000 | 10 |
| 1 bar | 100 | 1 |
| 1 mbar | 0,1 | 0,001 |
| 1 atm | 101,32500 | 1,01325 |
| 1 mH2O | 9,80665 | 0,0980665 |
| 1 mmHg | 0,133322368 | 0,00133322368 |
| 1 psi | 6,89475729 | 0,0689475729 |
| 1 inH2O | 0,249082 | 0,00249082 |
| 1 kg/cm² | 98,0665 | 0,980665 |
How the unit of pressure refers to the type of pressure
Expressing a pressure with a unit in its basic notation, such as Pa, bar of psi, doesn’t make much sense if you don’t know which type of pressure is referred to.
Sometimes you can guess the type of pressure based on the context, but usually, doubts will remain. If you have guessed incorrectly, serious errors may occur.
It is therefore always a good practice to indicate the type of pressure after the unit, meaning that the words ‘absolute’, ‘gauge’ or ‘differential’ should be written after the unit of pressure. Pressure could then be expressed as e.g. bar gauge or psi absolute.
Often, you will find a pressure unit followed by a suffix such as ‘g’, ‘a’ or ‘d’ (or written in capital letters) as in barg, psia or kPaD where ‘g’ stands for gauge, ‘a’ for absolute and ‘d’ for differential. The suffix is also sometimes noted in parentheses, e.g. bar(g).
Although these suffixes are still widely used, they are deprecated and no longer supported by international standards.
Related topics
Leave a Comment
Your email address will not be published. Fields marked with * are required.