8 unique temperature scales you may or may not know
Origin of temperature units
1 July 2018
We all use temperature scales every day, whether it’s just to know the outside temperature or to adjust the temperature in our room.
Dealing with temperature is so obvious that we no longer think about it.
But did you know that there were once 8 different, popular temperature scales to choose from?
And there were even more than 30 if you also count the less popular ones.
Fortunately, we don’t have to use all of them. Some are not in use anymore and others are only used for specific applications.
You could wonder why we have all these different temperature scales. Wouldn’t it be much easier to have only one?
Well, not really. There are different temperature scales for a reason. I tell you why at the end of the article.
Let’s first have a look at these 8 temperature scales.
The Newton scale (1701)

You must surely know Sir Isaac Newton (°1643 – †1727) or at least heard about him before.
There is a very famous story about him saying that he discovered his theory of gravitation when he sat down under an apple tree and an apple fell on his head.
If this is completely true, I don’t know, but he certainly discovered gravity and much more than that.
Newton was an English mathematician, astronomer, theologian, author, and physicist. One of the greatest scientists ever, comparable to Einstein, with discoveries like the color spectrum (using a glass prism) and the three laws of motion.
Next, to all these areas of interest, he was the first to develop a temperature scale in 1701.
With his thermometer made of a thick glass tube, 1.5 to 2 inches in diameter, and filled with linseed oil, he measured the temperature of melting snow. This temperature became his first defined point on the temperature scale. So he has assigned it the number 0.
The second defined temperature point on the Newton scale was body heat which he described as ‘the external heat of the body in its natural state’. He defined this temperature as 12 degrees of heat.
Yes, Newton didn’t speak about temperature, although he called his instrument a thermometer,
So now that he had calibrated his thermometer, he could start measuring temperatures, but only in the lower range because linseed oil reaches its boiling point at about 287°C (depending on the purity). Secondly, the oil starts to decompose around its boiling point which would change its coefficient of expansion, making the thermometer useless.
While his linseed thermometer would also be able to measure small negative temperatures, Newton never explored this side of the temperature scale. So the initial Newton scale had no negative numbers.
Newton didn’t have the intention to invent the thermometer for everyday use. As he was appointed Warden of the Royal Mint, a company that produced coins for the United Kingdom, he was more interested in determining the boiling points of metals.
To calibrate his thermometer for temperatures above body heat, he heated a ‘pretty thick piece of iron’ to the point where it became red hot.
He described the temperature of red hot iron as ‘the degree of heat of live coals in a small kitchen fire, made up of bituminous pit coals, and that burn without using bellows’.
Not a very accurate description of a temperature if you ask me, but that didn’t really matter because his intention was to calculate that temperature using his law of cooling and the already, for low temperatures, calibrated thermometer.
So, to do that, he placed small pieces of different metals on his red hot iron slab. These metals were fusible alloys of tin, lead, and bismuth, which melted fairly quickly by the heat of the iron block.
As the iron cooled down, the alloys solidified one by one at their own specific temperature.
Newton recorded the time when the iron block started to cool and also the times at which the different alloys solidified. Finally, he also noted the time when the iron had cooled down so much that its temperature could be measured directly with the linseed oil thermometer. This means that it had to cool down to body temperature.
Now he could apply the law of cooling to calculate the temperatures at which the alloys had solidified and the temperature of the iron at the start of the experiment.
He found 7 new defined points on his temperature scale:
| 40°N: | melting point of an alloy of one part lead, four parts tin and five parts bismuth |
| 48°N: | melting point of an alloy of equal parts of bismuth and tin |
| 57°N: | melting point of an alloy of one part bismuth and two parts tin |
| 68°N: | melting point of an alloy of one part bismuth and eight parts tin |
| 81°N: | melting point of bismuth |
| 96°N: | melting point of lead |
| 192°N: | heat of iron glowing as brightly as possible |
If you have never heard of the Newton temperature scale, no problem, it is no longer used.
| Convert Degrees Newton to | |||||||||||||||||
|
|
||||||||||||||||
The Rømer scale (1701)

Ole Christensen Rømer (°1644 – †1710) was a Danish astronomer who studied in Copenhagen (Denmark) and later went to work in Paris.
He also worked closely with astronomers such as Gottfried Leibniz, Christiaan Huygens, and Isaac Newton.
In 1676 he was the first to calculate the speed of light by making use of his observations of the eclipses of Io, a moon of Jupiter.
He found that light travels at a speed of 225.000 km/s.
Well, not all that accurate, since we now know it is 299.792,458 km/s (in a
When he returned from Paris, in 1681, he became a professor of astronomy at the University of Copenhagen and was appointed as director of the Roundtower Observatory a few years later.
In his own house, he had constructed his transit instrument, which he used to measure the position of stars, but the instrument was affected by the refraction of light and the changes in temperature.
To compensate for variations in temperature, he needed a thermometer.
Not an easy task to find a good thermometer at the time. Even if you found one, it had no temperature scale. Let alone that it could be used for scientific purposes.
So he decided to create one on his own and started to do some experiments with a glass tube filled with ‘spiritus vini colored with saffron’.
Yes, that means he used wine as a thermometer liquid.
This had some advantages. As wine is largely a mixture of water and alcohol, it had a more linear dilatation curve than pure water. Remember that water has a little odd behavior around 4°C.
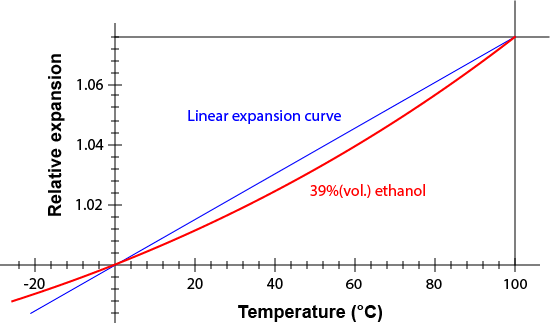
From calculations, it could later be found that the mixture contained 39% alcohol by volume.
Wow, they had strong wine back then. No wonder his calculation of light speed was a little off.
At this point, he had the glass tube and the fluid to put in.
Now, how did he calibrate his thermometer?
Well, when he realized that the air pressure above the liquid would have an influence on the height of the liquid column, he sealed the glass tube at the top.
Then he immersed the bulb of his thermometer in ice water and put a mark on the glass tube at the top of the liquid column.
After that, he submerged the bulb in boiling water and made another mark on the tube.
So now his thermometer was calibrated. The only thing left was to put numbers on the scale.
He didn’t want negative numbers because this would be too confusing and people would perhaps forget to put the minus sign in front of the number when writing down temperatures.
In order to avoid this, he divided his scale into 7 equal parts and also placed 1 equal part below the mark of the freezing point of water, so that the scale consisted of 8 equal parts in total.
He wrote the number 0 at the bottom of the scale and 60 at the top. Between these two marks, he made a linear distribution of degrees. According to this scale, the freezing point of water was at 7,5°Ro.
The fact that he chose the number 60 for the boiling point of water was not all that strange.
Being an astronomer he was used to using the sexagesimal (base 60) numeral system for performing calculations and measuring time, angles, and geographic coordinates.
This was the first thermometer that could be calibrated over and over again with high precision as the boiling and freezing points of water are easy to reproduce.
Nevertheless, as the years went by, better solutions emerged and the Romer temperature scale became out of use.
| Convert Degrees Rømer to | |||||||||||||||||
|
|
||||||||||||||||
The Fahrenheit scale (1724)

Daniel Gabriel Fahrenheit (°1686 – †1736) was born in Danzig (now Gdansk – Poland) as a child of prosperous parents.
He would have studied medicine if not both his parents had suddenly died from mushroom poisoning when he was 15 years old.
The foster parents, where he and his two brothers and two sisters were housed, were not in
Fahrenheit, however, was more interested in instruments than bookkeeping. So it was not long after his studies that he started making his own thermometers, the old-fashioned way with divisions in the same way as Florentine thermometers:
- The upper limit of 90° for ‘extreme heat’
- 0° for ‘moderate heat’
- The lower limit of 90° for ‘extreme cold’
In 1708 he went to Denmark to visit Rømer. It turned out to be a momentous encounter that had a major impact on his later work.
He learned from Rømer that good, consistent thermometers were in considerable demand for scientific work. But they needed to be identical to each other so that the measurements could be compared.
From then on he was convinced that a thermometer needed an accurate temperature scale and that this scale had to be reproducible.
He took the idea of a temperature scale from Rømer but did not like the decimals on the scale, e.g. 7,5° for freezing water and 22,5° for body heat.
So he rounded them up to 8 and 24, thereby using the freezing point of water and body heat as calibration points.
It is this scale that is, in fact, the original Fahrenheit scale. He changed it a couple of times later on for various reasons.
The exact moment at which he made these changes is not entirely clear because Fahrenheit did not keep notes of his experiments. All information we have comes from
At some point in time, it must have been around 1713, he multiplied all the numbers on his scale by 4, giving the scale a range of 96°F with the freezing point of water at 32°F.
This allowed the scale to be read more accurately because there were finer subdivisions that came in handy when using his thermometers for scientific purposes.
Another reason could have been that the 64° between his two calibration points (96° and 32°) is much easier to devise in equal parts, as 64 is the eighth power of 2.
So by consecutive divisions by 2 of each scale section, he could easily put the marks on his scale.
Some say that he used brine, a mixture of salt and water, which he cooled down to its freezing point and used this as the calibration point for 0°F.
But this can not be proven and is highly doubtful as different mixtures have been found in the literature and none of them freezes at exactly 0°F.
It is more likely that he used the brine as a checkpoint for his thermometer but calibrated it with freezing water.
Fahrenheit used alcohol as a thermometric liquid but was actually not satisfied with it because alcohol with exact same composition was difficult to obtain and therefore the expansion coefficient was always different.
Secondly, the boiling point of alcohol is low, so it’s impossible to measure high temperatures.
To overcome this problem he started new experiments with different thermometric liquids to finally discover that mercury was the ideal solution.
Fahrenheit was the first to use mercury as a thermometric liquid.
With mercury, he could make thermometers that could measure up to 600°F.
Further advantages of mercury are:
- Low freezing point: -38°C
- High boiling point: > 380°C
- Same expansion coefficient at all temperatures
- Doesn’t evaporate
- The silver color is easy to see
We now know that mercury is also toxic and therefore it is forbidden to use it.
Around 1713 he stops using body temperature or ‘blood heat’ as a calibration point because he found that it was very unreliable.
That was when he discovered that the body temperature of youngsters was higher than that of elderly people.
Instead, he used the boiling point of water as the top calibration point of his scale. This would then correspond to 212°F.
He had a mercury thermometer calibrated at the freezing- and boiling point of water that he used as his standard thermometer to which he compared all other thermometers he made.
Fahrenheit, who never had higher education, did not quite understand the mathematics of his inventions but his perseverance and constant experimentation helped him to build accurate measuring instruments.
After traveling around for a number of years, he finally settled in Amsterdam in 1717 and made thermometers, barometers, and aerometers.
He eventually died penniless in 1736 after all the money, from the inheritance of his parents and the money he earned, was spent on experiments.
| Convert Degrees Fahrenheit to | |||||||||||||||||
|
|
||||||||||||||||
The Réaumur scale (1731)

René Antoine Ferchault de Réaumur (°1683 – †1757), born in La Rochelle (France), was a French entomologist and writer.
He studied philosophy, civil law, and mathematics and went to Paris in 1703 to continue his studies of mathematics and physics.
In 1708, at the age of 24, he was elected a member of the ‘Académie des Sciences’ which was an institution promoting French scientific research.
During his life, he wrote a lot of scientific papers dealing with many branches of science, ranging from geometry to the forms of birds’ nests and everything in between.
As an entomologist, he observed insects which led to a number of findings such as the possibility of spiders being used to produce silk, or the process of wasps making paper from wood fibers to build their nests.
Because precise temperature measurements are important in the field of physics and the thermometers that were used for this purpose were usually not very accurate, he developed his own thermometer in 1731.
Réaumur wanted to produce comparable thermometers that could be used for scientific research.
He defined his thermometer with only one fixed point, the freezing point of water, which he marked with 0 degrees on his temperature scale.
As a thermometer liquid,
In this way, there was a
Réaumur carefully selected the composition of alcohol and water so that the thermometric liquid expanded 8% in volume when heated from the freezing point of water to the boiling point of water.
This means that 1000 parts at the freezing point of water gave 1080 parts at the boiling point.
This resulted in the definition of the boiling point at 80°Ré.
When applying a linear division between 0 and 80°, he ignored the non-linearity of the expansion of liquids and did not take into account the influence of atmospheric pressure, as a result of which Réaumur did not achieve his goal of producing comparable thermometers.
In addition, the thermometers were not suitable for measuring higher temperatures because alcohol has a low boiling point. This made them unsuitable for many applications.
However, his thermometers were used widely in Europe. Most of all in France, Germany, and Russia.
Around 1790, France introduced the metric system and therefore opted for the Celsius scale, but the Réaumur scale remained in use in some parts of Europe until the mid-19th century. In Russia even until the early 20th century.
In fact, even today in some parts of the food industry, the Réaumur scale is still used. For measuring the temperature of milk during cheese production in Switzerland and Italy for example, or for cooking sugar syrup for the production of meringue in some countries of Western Europe.
| Convert Degrees Réaumur to | |||||||||||||||||
|
|
||||||||||||||||
The Delisle scale (1732)

Joseph-Nicolas Delisle (°1688 – †1768) was a French astronomer and cartographer.
Born in Paris, he initially followed classical studies, but soon moved to astronomy. He entered the French Academy of Sciences in 1714.
In 1725, the Russian czar Peter the Great called him to Saint Petersburg to create and run the school of astronomy. He only arrived there in 1726. By that time the
In 1740 Delisle undertook an expedition to Siberia to observe the transit of Mercury across the sun. He set out from Saint Petersburg on 28 February 1740, arriving in Beryozovo (Siberia) on 9 April. A journey of 42 days.
But, he had no luck. All his efforts were in vain because on 22 April, the date of the transit of Mercury, the sun was obscured by clouds. Delisle was unable to make any astronomical observations.
Throughout the expedition, however, he made numerous other scientific observations about vegetation and wildlife in Siberia.
Despite all his efforts in the field of astronomy, he became best known for his invention of the Delisle temperature scale.
He made his first thermometers in 1724, using
The temperature of the Paris Observatory cellars was the only fixed point that he used to calibrate his thermometers.
It might seem a little odd to choose the temperature of a random cellar as a fixed point, but at that time a lot of
As from 1732 he changed his fixed point to the boiling point of water and marked it with 0°.
But with
To solve this problem he had to change his thermometric liquid to mercury which has a boiling point way above the boiling point of water.
Originally the scale had 2400 divisions, appropriate to the winter in Saint Petersburg. Each division corresponded to one hundred thousandths of the contraction of the mercury in the thermometer, with higher values at lower temperatures.
This means that the Delisle scale was an inverted scale, on which increasing cold was indicated with higher numbers. This had the advantage that, in a large area of application, the numbers were never negative.
In 1738 the scale was slightly recalibrated by Josias Weitbrecht, a German professor of medicine and anatomy. He kept the 0° as the boiling point of water but assigned a value of 150° as the freezing point of water.
The Delisle scale was in use in Russia for almost 100 years during the eighteenth and nineteenth centuries.
| Convert Degrees Delisle to | |||||||||||||||||
|
|
||||||||||||||||
The Celsius scale (1742)

Anders Celsius (1701-1744) was a Swedish astronomer and physicist who was born on November 27, 1701, in Uppsala, Sweden.
He was a professor at the University of Uppsala where he was teaching astronomy since 1730.
As an astronomer, he analyzed the changes in the Earth’s magnetic
In 1741 he founded the Observatory in Uppsala together with a few other people but he became best known for his invention of the Celsius scale in 1742.
Celsius created his temperature scale with only 2 defined points based on the physical characteristics of water.
The zero point (0°C) was defined at the boiling point of water and the second point (100°C) was defined at the freezing point of water, both referenced to the standard atmospheric pressure.
Yes, this is exactly the opposite of the Celsius scale as we know it today.
The reason why he chose an upside-down scale was that
Choosing the 100°C at the freezing point of water avoided negative numbers when it was freezing outside.
For people today this could feel a little awkward having increasing numbers for decreasing temperatures, but in the end, it all comes down to a habit.
Several years after the Celsius scale was invented, some scientists began to use the inverted scale with 0°C defined at the freezing point of water and 100°C defined at the boiling point of water.
Especially biologists found it interesting to invert the scale. Since plants are at risk of dying at 0°C because the water is frozen, the scientists found it more obvious to indicate temperatures below the freezing point of water with negative numbers and temperatures above with positive numbers.
It is not really known who was the first person to invert the scale. Some say it was Carolus Linnaeus (Linné), a professor of medicine and head of the botanical garden at Uppsala University, others think it was Martin Strömer who succeeded the late professor A. Celsius on the chair of astronomy.
Because of the 100-degree interval, Celsius originally called it the Centigrade scale,
It was the ‘Conférence Général des Poids et Mesures’ that decided to change the name because ‘grade’ was already in use as a unit of measurement and could have been confused with ‘centigrade’.
Celsius didn’t get very old. He died from tuberculosis in 1744 at the age of 42.
| Convert Degrees Celsius to | |||||||||||||||||
|
|
||||||||||||||||
The Kelvin scale (1848)

William Thomson, 1st Baron Kelvin (°1824 – †1907) and also called Lord Kelvin, was a Scots-Irish mathematical physicist and engineer.
He was born in Belfast (Northern Ireland) and worked at the U
Together with a lot of other scientists, he played an important role in the formulation of the first and second laws of thermodynamics.
In 1866 he was knighted by Queen Victoria for his theoretical work on submarine telegraphy and his inventions for use on submarine cables used during the transatlantic telegraph project. He received the title of honor ‘Sir’ to put in front of his name: Sir William Thomson.
For his achievements in thermodynamics, and for his opposition to self-government for Ireland within the United Kingdom of Great Britain, he was ennobled in 1892, becoming Baron Kelvin of Largs (a town in Scotland). Kelvin is the name of a river that flows close to his laboratory at the University of Glasgow.
William Thompson became the first British scientist who joined the House of Lords in England. Therefore he is also called Lord Kelvin. Lord is a title in the UK formally given to a baron or a member of the House of Lords.
Kelvin, like many
In other words, everything is frozen solid and there is no movement of atoms anymore.
In 1848, he published a paper, On an Absolute Thermometric Scale, that stated that this absolute minimum temperature was -273°C. Now, this value has been corrected to -273,15°C.
This means that negative temperatures on the Kelvin scale are impossible as nothing can be colder than the absolute zero temperature.
The divisions on the Kelvin scale are of exactly the same size as those of the Celsius scale, but the Kelvin unit is not expressed in degrees and thus the symbol ° is not present.
While relative temperature scales, e.g. Fahrenheit or Celsius, are comparing the temperature of an object to a randomly chosen fixed point (like the freezing point of water), absolute temperature scales work differently. They indicate temperatures compared to absolute zero.
In this way, absolute temperature scales are not only indicating the temperature of an object but also provide information about the amount of kinetic energy of its atoms and molecules. The kinetic energy is zero at a temperature of zero Kelvin and has a higher value at higher temperatures.
In 1954, the General Conference on Weight and Measures (CGPM) adopted the Kelvin as the base unit for thermodynamic temperature and formulated a definition for the unit:
The Kelvin, unit of thermodynamic temperature, is the fraction 1/273,16 of the thermodynamic temperature of the triple point of water.
As the triple point of water (273,16 K) is a very precise and reproducible temperature, it has been chosen as a fixed point on the Kelvin scale.
The water used for calibration of temperature measurements is not just any water. It has been standardized by the International Atomic Energy Agency (based in Vienna) in 1968 and is referred to as Vienna Standard Mean Ocean Water (VSMOW). The designation ‘ocean water’ refers only to the evaporation of ocean waters in the water cycle as the original source of fresh surface and ground waters.
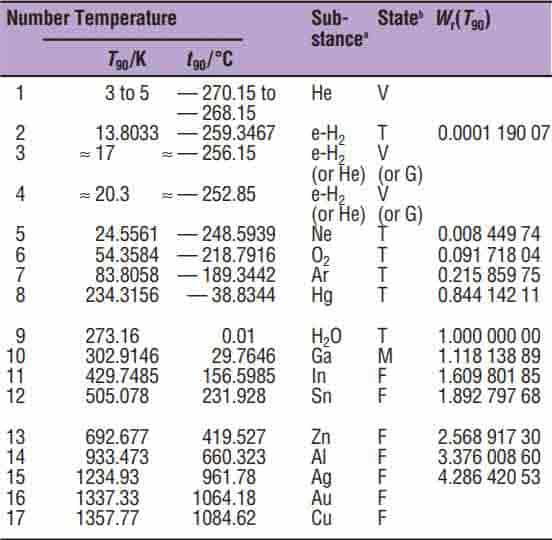
Besides the triple point of water, another 16 defining points have been selected by an international agreement, ranging from the freezing point of helium to the freezing point of copper.
Unless you are a scientist or an engineer, you will make little use of the Kelvin scale since it is used almost exclusively for scientific purposes.
| Convert Kelvin to | |||||||||||||||||
|
|
||||||||||||||||
The Rankine scale (1859)

William John Macquorn Rankine (°1820 – †1872) was a Scottish professor of Civil Engineering and Mechanics with a great interest in railway engineering, molecular physics, and thermodynamics.
He was born in Edinburgh (Scotland) but moved to Glasgow in 1851 to work at the University of Glasgow where he taught the theory and practice of civil and mechanical engineering.
Rankine was especially interested in thermodynamics and developed a complete theory of the steam engine and in fact of all heat engines.
He had also plenty of other interests in other branches of science, mathematics, and engineering.
In 1859 he developed his temperature scale. It was a thermodynamic scale just like the one Kelvin had developed, but the Rankine scale was based on degrees Fahrenheit instead of degrees Celsius, as was the case for Kelvin.
The Rankine temperature scale starts at absolute zero and every division has the same size of a degree Fahrenheit. Thus a temperature of 0 R is equal to 0 K, or -459,67 °F.
The triple point of water, which is at 491,688 R, is used as a fixed point on the scale. The water used here is also according to the Vienna Standard Mean Ocean Water (VSMOW) standard.
Most people write the Rankine unit as °R, but the US National Institute of Standards and Technology (NIST) is recommending against the use of the degree symbol to emphasize the similarity with Kelvin.
The Rankine temperature scale is only applied for scientific applications when formulas are expressed in Imperial Units. It can be used for calculations like radiation heat transfer, entropy change, the Carnot Heat Engine thermal efficiency, or the Ideal Gas Law.
The Rankine scale has been largely replaced by the Kelvin scale. Nowadays, I imagine it is hard to find any scientific work making use of the Rankine unit. But, if you want to dive into old scientific papers, knowledge of this temperature scale can come in handy.
Rankine, unmarried and without children, died on Christmas Eve in 1872 at the age of 52.
| Convert Rankine to | |||||||||||||||||
|
|
||||||||||||||||
Why do we have different temperature scales?
As I promised you at the start of this article, let me explain to you why we have different temperature scales.
Back in history, in the 18th and 19th centuries, there was a big discussion among scientists about the best way to measure temperature.
First of all, which thermometric liquid should be used? Secondly, how to graduate the scale? And thirdly, how to calibrate it?
A lot of experiments were going on and some scientists, often astronomers who had to compensate their measurements for variations in temperature, started to make their own thermometers.
Some of them did a good job and succeeded in creating a reliable thermometer. Others did not succeed and their thermometers quickly went into oblivion.
So, there was and still is historical evolution in using temperature scales. We didn’t create just one scale, we created many different scales.
Some of these scales were widely used in Europe and Russia.
Changing from one scale to another takes time, time to convince people to use the other scale. As you know, people always resist change.
Temperature scales, like those created by Newton, Rømer, Réaumur, or Delisle, all disappeared and were replaced by the Celsius scale when a lot of countries started to introduce the metric system around 1790.
A few countries maintained the imperial or the customary system and continued to measure temperature in degrees Fahrenheit. Today, these countries are the United States, Liberia, and Myanmar. Some other countries use both Fahrenheit and Celsius.
The Kelvin and Rankine scales are only used for scientific reasons, whereby Kelvin is by far the most used scale, and Rankine is only used in a few specific fields of engineering, like thermal power plants.
So, this leaves us with only 3 different scales which are Fahrenheit, Celsius, and Kelvin. Knowing that the Fahrenheit scale is in decay, there may be only two scales in the future.
Eventually, perhaps only Kelvin will remain, though it still sounds a bit weird to indicate outside temperature with 3 digits.
References
- Wikipedia. 2018. Isaac Newton.
- Wikipedia. 2018. Newton scale.
- Rowlands Peter. 2018.Newton – Innovation And Controversy. World Scientific Publishing Europe Ltd. 310pp.
- Live Science. 2013. Celsius: Facts, Formulas & History.
- Uppsala Universitet. 2001. History of the Celsius temperature scale.
- Rundetaarn. Early Danish thermometers.
- Wikipedia. 2016. Ole Rømer.
- Pieter van der Star. 1983. Fahrenheit’s letters to Leibniz and Boerhaave. Rodopi B.V. 199pp.
- Science Alert. 2016. Watch why people still use this crazily arbitrary temperature scale.
- Deutsches Thermometermuseum Geraberg. René-Antoine Ferchault de Réaumur.
- Académie des sciences. René Antoine Ferchault de Réaumur.
- Wikipedia. 2018. Joseph-Nicolas Delisle.
- Jan Gyllenbok. 2018. Encyclopaedia of Historical Metrology, Weights, and Measures, Volume 1. 676pp.
- Sofia Talas. 2002. J.B. Micheli du Crest’s Thermometer and The Connections with G.F. Brander. Scientific Instrument Society. 72: 20-25.
- Harold I. Sharlin. Encyclopaedia Britannica. 2018. William Thomson, Baron Kelvin – Scottish engineer, mathematician, and physicist.
- National Institute of Standards and Technology. 2018. Kelvin: Introduction.
- University of Glasgow. Macquorn Rankine.
Related topics
4 Comments
Leave a Comment
Your email address will not be published. Fields marked with * are required.














I have read so many posts about the blogger lovers however this post is really a good piece of writing, keep it up
Thanks so much, Rama.
I really enjoy the blog article. Much obliged.
I’m glad you enjoyed it.