Mercury vs alcohol thermometer
Why mercury is used in thermometers?
1 July 2018

In the early days of thermometry, there was a lot of doubt about what thermometer liquid to use. Should they have used alcohol or was it better to use mercury?
Both liquids have their set of advantages.
Let’s list them up.
Alcohol thermometers
Advantages of alcohol (ethanol):
- It has a very large thermal expansion coefficient.
- Unharmful to humans and the environment (at least if you don’t drink it!).
- A low freezing point (-115°C) makes it possible to measure very low temperatures.
- It’s a bit cheaper than mercury.
Disadvantages of alcohol (ethanol):
- Impossible to measure high temperatures because of the low boiling point of alcohol.
- It has a nonlinear expansion coefficient which leads to a small measurement error.
- Capillary separation (interrupted alcohol column).
- Alcohol is colorless, so dye (blue or red) must be added to make it visible.
The advantage of alcohol (ethanol) is that it has a very large thermal expansion coefficient.
This results in a large change of the liquid column inside the capillary of the thermometer and contributes to the accuracy of the measurement.
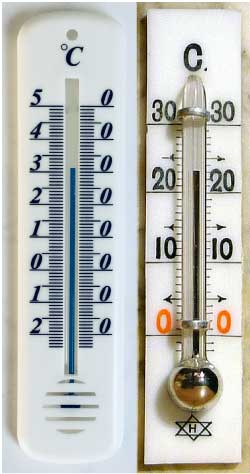
Right: mercury thermometer
Mercury thermometers
Advantages of mercury:
- Mercury thermometers give accurate readings.
- Quick reaction to changes in temperature because metal is a good heat conductor.
- Mercury has a very low saturation vapor pressure.
- Possible to measure higher temperatures as in cooking.
- Same expansion coefficient at all temperatures.
- The shiny silver color is easy to see.
Disadvantages of mercury:
- Mercury is toxic, especially in a gaseous state.
- It’s a bit more expensive than alcohol.
- Low thermal expansion coefficient.
I guess the advantage of a very low saturation vapor pressure of mercury needs a little more explanation.
So, if we consider a perfect thermometer, the volume above the liquid should be a vacuum.
In reality, however, this isn’t the case. There will be always a small number of gas molecules present in the volume above the liquid.
Since ethanol evaporates faster than mercury, more gas molecules will be present in that volume.
This results in a pressure build-up above the liquid surface and a faulty indication of the thermometer.
CONCLUSION:
Whether the best thermometer liquid is alcohol or mercury depends on the application.
When the thermometer is used to measure the outside temperature, alcohol will do fine.
The readout will be sufficiently accurate, even with a nonlinear expansion coefficient, and it will still work at very cold temperatures.
If the purpose of the thermometer is to use it during the preparation of food, mercury could be used because of its high boiling point (356,7°C).
If alcohol was used for this application, it would start to boil at around 78°C. The vapor pressure would rise quickly inside the capillary and in the end, the bulb will break and spoil your dinner.
Important to know however is the fact that mercury is toxic and the use of mercury-in-glass thermometers is forbidden in many countries, except for industrial and scientific applications.
For reasons of toxicity alone, I would not recommend using mercury in food preparation. Glass thermometers can easily break. A better choice would be to use a digital thermometer.
Mercury is perhaps the best thermometric fluid, but due to its poisonous property, it is now increasingly being banned from laboratories and industry in favor of alternative methods.
These alternatives include:
- Resistance Temperature Detector (RTD) (-200 / +850°C)
- Platinum Resistance Thermometer (PRT) (-200 / +850°C)
- Thermistor (-50 / +250°C)
- Thermocouple (-250 / +3000°C)
- Organic-Liquid-Filled thermometers (-100 / +100°C)
Related topics
1 Comment
Leave a Comment
Your email address will not be published. Fields marked with * are required.













This explanation is very helpful thanks