Pressure units converter
Convert bar to psi, psi to bar, MPa to psi …
10 July 2019
UnitConversion.org – the universal assistant for all of your unit conversion needs.
Other unit converters
How to use the Pressure Units Converter
- Select the unit you want to convert from in the input unit list on the left.
- Select the unit you want to convert to in the output unit list on the right.
- Enter the value of the input unit to convert from in the input box on the left.
- The tool will immediately display the equivalent value of the output unit in the output box on the right.
Steps 1 to 3 may be executed in random order.
The value of your input unit can be converted to any other unit by just selecting a different output unit in the list on the right.
The most frequently used pressure units
The world’s most frequently used pressure units are:
- pascal (Pa): this is the SI unit
- kilopascal (kPa)
- megapascal (MPa)
- pound per square inch (psi)
- atmosphere (atm)
- torr (mmHg)
- bar
Pressure conversion factors
The conversion factors to convert between the most frequently used pressure units can be found in the table below:

| CONVERT TO (multiply by) | |||||||
| pascal | kilopascal | megapascal | pound square inch | atmosphere | torr | bar | |
| CONVERT FROM | (Pa) | (kPa) | (MPa) | (psi) | (atm) | (mmHg) | (bar) |
| pascal (Pa) | 1 | 103 | 106 | 1.4503×10-4 | 9.8692×10-6 | 7.5006×10-3 | 10-5 |
| kilopascal (kPa) | 10-3 | 1 | 103 | 0.1450 | 9.8692×10-3 | 7.5006 | 10-2 |
| megapascal (MPa) | 10-6 | 10-3 | 1 | 145.0377 | 9.8692 | 7500.6168 | 10 |
| Pound square inch (psi) | 6894.7572 | 6.8947 | 6.8947×10-3 | 1 | 0.0680 | 51.7149 | 6.8947×10-2 |
| atmosphere (atm) | 1.0132×105 | 1.0132×102 | 0.1013 | 14.6959 | 1 | 760 | 1.013 |
| torr (mmHg) | 133.3223 | 0.13332 | 1.3332×10-4 | 1.9336×10-2 | 1.3157×10-3 | 1 | 1.3332×10-3 |
| bar | 105 | 102 | 0.1 | 14.5037 | 0.9869 | 750.0616 | 1 |
Some factors are slightly rounded compared to the factors used in the Pressure Units Converter.
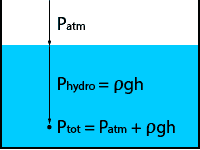
Manometric units of pressure
Units like millimeters of mercury or centimeters of water are called manometric units. These units depend on an assumed density of a fluid and an assumed acceleration due to gravity.
Most of these units are based on the following assumptions:
- standard gravity at sea level and 45° latitude:
- 9.80665 m/s2 (32.17405 ft/s2)
- density of pure water at one of below temperatures:
- 4°C (39.2°F) = 1000 kg/m3 (1.9403 sl/ft3)
- 60°F (15.6°C) = 1.9385 sl/ft3 (999.07 kg/m3)
- density of pure mercury at one of below temperatures:
- 0°C (32°F) = 13595.1 kg/m3 (26.3788 sl/ft3)
- 60°F (15.6°C) = 26.3071 sl/ft3 (13558.14 kg/m3)
Manometric units must be used with caution as they are influenced by latitude and temperature.
Because the shape of the Earth is not a perfect sphere but is flattened at the poles, the acceleration due to gravity depends on latitude. The Earth’s radius is smaller at the poles than at the equator. Therefore, gravity is greatest at the poles and least at the equator.
When these units are used under non-standard conditions of latitude and temperature, a correction must be applied to convert the measurement result to standard conditions.
National standard organizations discourage the use of manometric units because of the complexity that results from converting to standard conditions as well as the existence of different conventions used for the temperature and density of the liquid.
Leave a Comment
Your email address will not be published. Fields marked with * are required.